Theme: Promulgating the prevention of adverse drug reaction
Global Pharmacovigilance-2019
Conference Series llc LTD warmly invites all the participants across the world to “13th Global Pharmacovigilance & Clinical Trail Summit” scheduled on March 28-29, 2019 Osaka, Japan. This conference provides a forum for interaction among attendees with discussion involving discovery of a new drug, Challenges in drug development, Pre-Clinical and Clinical trial reports, Case Studies, Pharmacovigilance, Biosimilar and Biologistics and its eye on new drug regulatory approvals. Session discussions focus on discussions about approaches and innovations for Patient Benefit Risk Management in Pharma, Biotech and Health care Industry.
This is a 2 day Mega Event offering Exhibition, at venue to showcase the new and emerging technologies with Keynote presentation, Oral talks, Poster presentations and Exhibitions.
Why to Attend???
To meet your targeted audience in the Series of Pharmacovigilance 2019 Conference with a largest assemblage of participants from the Pharma, Clinical, Healthcare and Regulatory community providing an international and non-biased platform for professionals working in early discovery and preclinical research to exchange ideas on best practice and share case studies on innovation within the area alarming on Medication Safety.
It is an Unique Opportunity for Advertisers and Sponsors at this International event.
Who to attend???
- Pharmacovigilance Students, Scientists
- Pharmacovigilance Researchers & Teachers
- Medical Colleges
- Pharmaceutical Industries
- Pharmacovigilance Associations and Societies
- Pharmacovigilance Training Institutes
- Software Developing Companies
- Medical Devices Manufacturing Companies
- Data Management Companies
- Business Entrepreneurs
Also, Directors/Senior Directors/Executive Directors and Vice Presidents/Senior Vice Presidents/Executive Vice Presidents and Heads/Leaders/Partners of:
- CROs and CMOs
- Clinical Research Sites
- Pharma/Biotech and Medical Device industries
- Hospitals, Associations
University Faculties scientists who are related to clinical and medical research like
- Directors
- Senior Professors
- Assistant Professors
- Associate Professor
- Research Scholars
- Ph.D Students
With the grand success of Pharmacovigilance Series of Conferences in UK, USA in consecutive years over the last several years which met with great achievement in Business Conferencing. It’s glad to announce 13th Global Pharmacovigilance & Drug Safety Summit during March 28-29, 2019 Osaka, Japan with the theme “Explore the emerging tools for process automation in pharmacovigilance”. This is a 2 day Mega Event offering Exhibition, at venue to showcase the new and emerging technologies with Keynote presentation, Oral, YRF (Student Presentation), poster, e-poster Presentations.
Track 1: Adverse Drug Effects
Disagreeable medicine reactions can be seen as a sort of noxious quality or overhauled calm effects that occur in the midst of appropriate use (when sedate absorption is by chance curbed by a disarray or another pharmaceutical). In the US, 3 to 7% of all hospitalizations are a result of troublesome solution reactions. ADRs occur in the midst of 10 to 20% of hospitalizations; around 10 to 20% of these ADRs are extraordinary. Rate of death on account of ADRs is dark; prescribed rates of 0.5 to 0.9% may be mistakenly high in light of the fact that a substantial number of the patients included had real and complex issue. Rate and reality of hostile prescription reactions vacillate by tolerant qualities (age, sex, ethnicity, existing together issue, genetic or geographic components) and by calm parts (sort of solution, association course, treatment term, estimations, and bioavailability). Event is higher with front line age and polypharmacy.
Related Societies and Associations:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, , International Pharmaceutical Federation, International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA),International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT), European Drug Utilization Research Group (EURODURG
Track 2: Causality Assessment
Causality assessment of ADRs is a method used for estimating the strength of relationship between drugs exposure and occurrence of adverse reactions. Causality assessment of ADRs may be undertaken by clinicians, academics, the pharmaceutical industry and regulators, and in different settings, including clinical trials. At an individual level, health-care providers assess causality informally when dealing with ADRs in patients to make decisions regarding future therapy. Many regulatory authorities assess spontaneous ADR reports, where causality assessment can help in signal detection and risk–benefit decisions regarding medicines, using formal Causality Assessment to aid in this process.
Related Societies and Associations:
Drug Information Association, International Pharmaceutical Federation, European Drug Utilization Research Group(EURODURG), International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry, Pharmaceutical Information and Pharmacovigilance Association ( PIPA), International Society of Pharmacovigilance (ISOP), The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT), International Epidemiology Association, European Association of Employed Community Pharmacists in Europe (EPhEU), Association of Pharmacy Professionals (APP)
Track 3: Clinical Trials Pharmacovigilance
Clinical trial safety is an important component of pharmacovigilance. Every medicinal product must have satisfactorily completed a clinical trial program establishing acceptable evidence of safety and efficacy before being placed onto the market. clinical trials have a regulatory definition states that any investigation in human subjects intended to discover or verify the clinical, pharmacological and/or other pharmacodynamic effects of one or more investigational medicinal products, and/or to identify any adverse reactions to one or more investigational medicinal product(s) and/or to study absorption, distribution, metabolism and excretion of one or more investigational medicinal product(s) with the objective of ascertaining its (their) safety and/or efficacy.
The exact nature of these pre-registration trials will depend on several factors including the drug itself; the particular disease or indication it has the potential to treat; and the patient group studied.
Related Societies and Associations:
International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research(ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics(EACPT), European Drug Utilization Research Group (EURODURG) International Epidemiology Association, European Association of Employed Community Pharmacists in Europe (EPhEU)
Track 4: Pharmacovigilance Practice
The piece of Good Pharmacovigilance Practice and Pharmacoepidemiology in Risk Management is prevalently to manufacture the probability of productive effects of a drug in a people than the probability of hostile effects and to keep up the Good Reporting Practices by avoiding the noteworthy issues in peril organization. Furthermore, it is basic to center around Signal examination by party the information on new or cloud solution impacts that is conceivably caused by a medicine and that finally should incite ensuring prosperity. The pharmacovigilance and clinical preliminaries organizations giving associations should have the Pharmacovigilance confirmation.
Related Societies and Associations:
Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT)
Track 5: Drug Safety
Prescription Safety is the pharmacological science ensuring security and related to the aggregation, area, assessment, watching, and repugnance of hostile side effects with pharmacological movement of pharmaceutical things. As demonstrated by US FDA a drug is seen as protected by looking, its collecting technique and delayed consequences of animal testing and clinical preliminaries. In this track, we look at Drug security and its applications in various fields, for instance, Software, Training et cetera.
Related Societies and Associations:
The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT), International Pharmaceutical Federation, International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research(ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA), International Society of Pharmacovigilance (ISOP)
Track 6: Clinical Trial Protocols
An exploration convention is a record that depicts the foundation, reason, goals, outline, philosophy, factual contemplations, and association of a clinical research venture. As per the ICH Good Clinical Practice rules, a convention ought to incorporate the accompanying points: Cover sheet (General Information), Foundation Information, Destinations/Purpose, Study Design, Choice and Exclusion of Subjects, Treatment of Subjects, Appraisal of Efficacy, Appraisal of Safety, Unfriendly Events, Stopping of the Study, Measurements, Quality Control and Assurance, Morals, Information taking care of and Recordkeeping, Distribution Policy, Task Timetable/Flowchart, References, Supplements/Appendices.
Related Societies and Associations:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA)
Track 7: Diversity in Industrial Clinical Trials and Clinical Research
The clinical preliminary industry is continually creating with improved clinical research headways and new clinical examinations are being moved at a reliably creating pace. Clinical preliminaries have constantly been a basic bit of the medicine change process, as they give clinical data on the best courses for treating over the top issue and illnesses. The criticalness of better than average assortment in mechanical clinical preliminaries and clinical research is to ensure that cutting edge clinical preliminaries are doing due steadiness and being as key as possible in their results.
Related Societies and Associations:
International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT), European Drug Utilization Research Group (EURODURG) International Epidemiology Association, European Association of Employed Community Pharmacists in Europe(EPhEU), Association of Pharmacy Professionals (APP), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM)
Track 8: Clinical Research and Statistics
In Clinical Research, Statistics expect a discernible part in managerial sections. Estimations associated with clinical research give formal accounting to wellsprings of change in patient's responses to treatment. The researchers make use of bits of knowledge to shape sensible and correct deriving from assembled data and to settle on exact decisions inside seeing vulnerability. True examination of Pharmacovigilance can be refined by a couple of principles fundamentally ICH rules. Ominous Drug Reactions reports can in like manner be considered for the managerial convenience.
Related Societies and Associations:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA)
Track 9: Clinical Database Management
There is good position in concentrating all prosperity data, clinical data, examination and uncovering with one provider. Pharmacovigilance Software mechanical assembly gives broad examination of disagreeable events rising up out of the usage of Pharmaceutical things (Medicinal Product, Medical Device, Vaccines, Non-Drug Therapy and Veterinary Medicinal Product). The pharmaceutical security database allows the risk advantage examination of therapeutic things thinking about new and creating information, with respect to add up to information. Pharmacovigilance since beginning has been a consistence driven development, wherein your managerial consistence chooses association's danger evaluation scores. A solution security database offers arranging of cautions for encouraged cases, follow-up cases and PSUR/PADER reports convenience to meet managerial course of occasions consistence.
Related Societies and Associations:
International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research(ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics(EACPT), European Drug Utilization Research Group (EURODURG) International Epidemiology Association, European Association of Employed Community Pharmacists in Europe (EPhEU)
Track 10: Hospital and Industrial Pharmacy
They are experts in the field of meds and are accountable for the allotting of cures and in addition the purchase, make and quality testing of all meds used as a piece of a mending focus. Various specialist's office tranquilize experts are possessed all the necessary qualities to suggest in their own particular right. Medication experts work personally with therapeutic and nursing staff to ensure that patients get the best treatment, provoking on the assurance, estimation and association course. They moreover give help and advice to patients in all parts of their remedies.
Mechanical Pharmacy moreover accept a fundamental part in any prescription divulgence. To any novel prescription disclosure, the mechanical approach is fundamental to get gigantic business application. Scarcely any things which must be considered by dares to give a safe and cost brimming with feeling answer for the patients like Supply chain, Waste organization, Product organization, Post-exhibiting surveillance, Good amassing practices and Marketing.
Related Societies and Associations:
Society for Clinical Data Management, International Pharmaceutical Federation, International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA), International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT), International Epidemiology Association, European Association of Employed Community Pharmacists in Europe (EPhEU)
Track 11: Analysis of Data quality and Management
Centralizing all safety data, clinical data, analysis and reporting with one provider is advantageous. Pharmacovigilance Software tool provides broad analysis of adverse events arising from the use of Pharmaceutical products (Medical Device, Medicinal Product, Veterinary Medicinal Product, Vaccines, Non-Drug Therapy. The drug safety database permits the risk- benefit analysis of medicinal and medical products taking into account, new and emerging information. Pharmacovigilance is a compliance driven activity, whereas regulatory compliance determines company’s risk assessment scores. A drug safety database provides follow-up cases, scheduling of alerts for expedited cases and PADER /PSUR reports submission to meet regulatory timeline compliance.
Related Societies and Associations:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA)
Track 12: PV Consultings And Business opportunity
Owing to the changing resources which are necessary to fulfil the regulatory requirements, few companies choose to outsource or out task regulatory affairs to service providers externally. Regulatory Affairs department is constantly evolving and growing and hence it is one which is least impacted during the Acquisition and Merger, and also during recession. The stringent regulations on safety monitoring and their periodical revision have led to increased safety in data collection, analysis, regulatory surveillance including costs. Thus, Pharmacovigilance has become a critical phase in clinical development programs.
Related Societies and Associations:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA)
Track 13: Biopharmaceutics
This is a main branch in Pharmaceutical Sciences which relates the physicochemical properties of the drugs - dosage form, pharmacology, toxicology / clinical response observed after its administration. Biopharmaceuticsuses the exact formulation to obtain the drug with new dosage form as related to the onset, duration, and intensity of drug action, including constituents and mode of manufacture.
Discussions under this track include rational drug management of cancer, diabetes and cardiovascular disorders, Management of psychiatric disorders and autoimmune disorders, Bioavailability and bioequivalence, drug disposition, Invivo-invitro correlation, Pharmacodynamics, Drug Interaction, Bio analytical method, Clinical Pharmacology, Clinical toxicology, Biomarkers, Recent Biomedical Innovation
Related Societies and Associations:
International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT), European Drug Utilization Research Group (EURODURG) International Epidemiology Association, European Association of Employed Community Pharmacists in Europe(EPhEU), Association of Pharmacy Professionals (APP), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM)
Track 14: Regulatory Affairs
Regulatory Affairs is involved in all the stages of development of a new medicine and its post-marketing activities with medicinal products authorities. This department heart of pharmaceutical industry. Internally it is a cluster of drug development, drug manufacturing, drug marketing and clinical research. Outward it is an interface between the company and regulatory authorities. In the Clinical trial approaches, major role is played by regulatory affairs for clinical trials. According to regulatory affairs guidelines clinical trials are to be conducted. Regulatory affairs are independent for specific country and their guidelines. International harmonization in principles has led to consistent approach in regulatory submissions and hence its review.Medication Regulations is a basic in part of Pharmaceutical sciences where it oversees Drug prosperity, Cost suitability, Drug rediscovery, Pharmaceutical organizations, Role of medication experts, Radio Pharmaceuticals and Multiple prescription use et cetera. The course of drugs moves by domain. In a couple of countries, for instance, the United States, they are overseen at the national level by a singular office. In various wards they are overseen at the state level or at both state and national levels by various bodies, like the case in Australia.
Related Societies and Associations:
International Society for Pharmacoepidemiology, Center for Drug Safety and Effectiveness, Center for Public Health and Human Rights, Center for Health Services and Outcomes Research, International Pharmaceutical Federation, International Society of Pharmacovigilance , Society for Clinical Trials, Association of Clinical Research, The Society of Clinical Research Associates, Clinical Research Society, American Association of Pharmaceutical Scientists, Association of British Pharmaceutical Industry , Indian Society for Clinical Organization Research (ISCR), Pharmaceutical Information and Pharmacovigilance Association ( PIPA),
International Society of Pharmacovigilance (ISOP), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT), European Drug Utilization Research Group (EURODURG) International Epidemiology Association, European Association of Employed Community Pharmacists in Europe(EPhEU), Association of Pharmacy Professionals (APP), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM)
Track 15: Pharmacovigilance Significance & Scope
Medical device Pharmacovigilance is monitoring of safety profile of medical devices, from the processing to reporting of single adverse incidents including the removal of products from the market as fragment of a Field Safety Corrective Action. This to ensure that patient’s and healthcare professional’s safety is monitored, and action taken as soon as a safety concern with a medical device arises.
Related Societies and Associations:
European Association of Employed Community Pharmacists in Europe (EPhEU), Association of Pharmacy Professionals (APP), Pharmaceutical Society Of New Zealand (PSNZ), Pharmaceutical Association of Mauritius(PAM), Pharmaceutical Information and Pharmacovigilance Association ( PIPA), Drug Information Association, The Australian Society of Clinical and Experimental Pharmacologists and Toxicologists (ASCEPT), European Association for Clinical Pharmacology and Therapeutics (EACPT), European Drug Utilization Research Group (EURODURG) International Epidemiology Association
Track 16: Entrepreneurs Investment Meet
A platform aimed to connect Entrepreneurs, Investors, and Proposers globally. It's intended to create and facilitate industrial delegates, research scientists, business delegate a viable meeting place for engaging people and it would be a good opportunity for Startup Company representatives in global business discussions, evaluation and execution of promising business ideas. For entrepreneurs, it would be an ideal place to find out suitable investors and partners to start and/or expand their business.
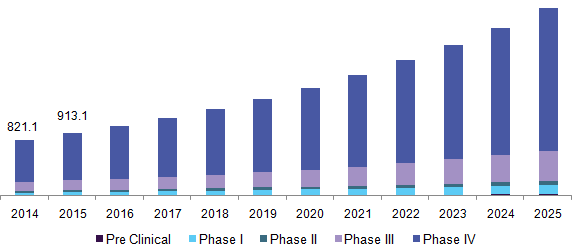
The global pharmacovigilance (PV) market is projected to reach 10.27 billion USD by 2025. The market is expected to see a growth at 13.1% CAGR owing to increasing incidence of Adverse Drug reaction. The key driver for the growth of pharmacovigilance market is ADR’s. In 2015, the Japan FDA received almost 253,017 serious adverse events and 44,693 deaths associated with adverse drug reactions (ADRs). This shows the possible demand for instigating safety and pharmacovigilance services.
Rising demand for drugs have been significantly amplified and the need for novel therapeutics development via extensive clinical trials, which is further expected to serve this market with lucrative opportunities. The largest share of the pharmacovigilance and drug safety software market in 2014. However, Asian and Latin American countries symbolize high growth markets. This is owing to a rise in research outsourcing by pharmaceutical giants and growing public and private investments in pharmaceutical R&D in these emerging nations.
Major pharmaceutical companies are involved in extensive R&D initiatives for development of innovative therapeutic molecules. This has resulted in increased rate of drug development. Manufacturers are now focusing on remodeling their product development processes in an attempt to cater to patient needs across the globe. These factors are anticipated to fuel the demand for PV services.
In April 2017, Accenture entered in a collaborative agreement with Bio Celebrate to develop a platform for aggregating and analyzing clinical information for improvement in drug developing efficiency, thus enhancing its R&D capabilities. These factors are anticipated to fuel the market growth. The largest share of the pharmacovigilance and drug safety software market in 2014. However, Asian and Latin American countries symbolize high growth markets. This is owing to a rise in research outsourcing by pharmaceutical giants and growing public and private investments in pharmaceutical R&D in these emerging nations.
Conference Highlights
- Clinical Research and Statistics
- Adverse Drug Reactions
- Causality Assessment
- Hospital and Industrial Pharmacy
- Clinical Trials Pharmacovigilance
- Pharmacovigilance Significance & Scope
- Pharmacovigilance Practice
- Drug Safety
- Clinical Trial Protocols
- Diversity in Industrial Clinical Trials and Clinical Research
- Clinical Database Management
- PV Consultings And Business opportunity
- Biopharmaceutics
- Regulatory Affairs
- Analysis of Data quality and Management
- Entrepreneurs Investment Meet
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | March 28-29, 2019 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | Day 2 | |
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Clinical Trials
- Journal of Pharmacovigilance
- Journal of Clinical & Experimental Pharmacology
Abstracts will be provided with Digital Object Identifier by





































