Theme: Present Challenges and Futuristic Scope in Pharmacovigilance and Clinical Trials
Global Pharmacovigilance-2020
Conference Series llc LTD warmly invites all the participants across the world to “14th Global Pharmacovigilance & Clinical Trail Summit” scheduled on March 16-17, 2020 Sydney, Australia. This conference provides a forum for interaction among attendees with discussion involving discovery of a new drug, Challenges in drug development, Pre-Clinical and Clinical trial reports, Case Studies, Pharmacovigilance, Biosimilar and Bio logistics and its eye on new drug regulatory approvals. Session discussions focus on discussions about approaches and innovations for Patient Benefit Risk Management in Pharma, Biotech and Health care Industry.
This is a 2-day Mega Event offering Exhibition, at venue to showcase the new and emerging technologies with Keynote presentation, Oral talks, Poster presentations and Exhibitions.
Why to Attend???
To meet your targeted audience in the Series of Pharmacovigilance 2020 Conference with a largest assemblage of participants from the Pharma, Clinical, Healthcare and Regulatory community providing an international and non-biased platform for professionals working in early discovery and preclinical research to exchange ideas on best practice and share case studies on innovation within the area alarming on Medication Safety.
It is an Unique Opportunity for Advertisers and Sponsors at this International event.
Who to attend???
Pharmacovigilance Students, Scientists
Pharmacovigilance Researchers & Teachers
Medical Colleges
Pharmaceutical Industries
Pharmacovigilance Associations and Societies
Pharmacovigilance Training Institutes
Software Developing Companies
Medical Devices Manufacturing Companies
Data Management Companies
Business Entrepreneurs
Also, Directors/Senior Directors/Executive Directors and Vice Presidents/Senior Vice Presidents/Executive Vice Presidents and Heads/Leaders/Partners of:
CROs and CMOs
Clinical Research Sites
Pharma/Biotech and Medical Device industries
Hospitals, Associations
University Faculties scientists who are related to clinical and medical research like
Directors
Senior Professors
Assistant Professors
Associate Professor
Research Scholars
Ph.D Students
Track 1: Pharmacovigilance and Clinical Trials
Pharmacovigilance and Clinical Trials will have the fast development of new drug and innovative therapeutics, new pharmacovigilance actions and methods have to be implemented for assurance and Patient Benefit Safety Management in Pharma, Biotech and Health Care. Clinical Trails contain the best quality level to calculate the viability and wellbeing of new medications. However, in light of the fact that they are directed in homogeneous conditions a long way from this present reality of solution and use, errors in patient choice or treatment situations may change both the viability and risks.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Pharmacovigilance Sydney | Pharmacovigilance Australia 2020 | Pharmacovigilance Sydney 2020 | International Pharmacovigilance sydney Australia | pharmacovigilance and clinical trials 2020 | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit
Track 2: Pharmacovigilance Practice
The piece of Good Pharmacovigilance Practice and Pharmaco-epidemiology in Risk Management is widely to manufacture the probability of productive effects of a drug in a people than the probability of antagonistic effects and to keep up the Good Reporting Practices by evading the important matters in peril organization. Moreover, it is basic to center around Signal examination by party the truths on new or cloud solution influences that is possibly produced by a medicine and that finally should incite ensuring prosperity. The pharmacovigilance and clinical preliminaries organizations generous associations should have the Pharmacovigilance validation.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Pharmacovigilance congress 2020 | Global Pharmacovigilance 2020 conference | Global Pharmacovigilance 2020 Conference | Global Pharmacovigilance 2020 summit | Global Pharmacovigilance 2020 Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference | Global Pharmacovigilance 2020 sydney | Global Pharmacovigilance sydney Australia | Global pharmacovigilance and clinical trials 2020 | Global Clinical Trials summit |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit
Track 3: Clinical Trials Pharmacovigilance
A clinical preliminary includes the testing of additional medication (or other treatment) to gauge whether it is influential and safe. Clinical preliminaries of medications can be divided into those surveying the treatment of the sickness (for example asthma) or those assessing medications to counteract the happening of huge medicinal services events later on (for example stroke). Clinical preliminaries take the quantitative data around the advantages, antagonistic influences and potential services of new medicates that license prescribers and patients to settle on sound choices with respect to tranquilize treatment.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit
Track 4: Adverse Drug Reactions
We rapid associate adverse drug reaction as “an noticeably damaging or unfriendly reaction, subsequent from associate interference associated with the service of a healthful product that forecasts hazard from future administration and warrants bar or exact treatment or change of the dose plan, or extraction of the merchandise.” Such responses are presently reportable by use of WHO's Adverse Reaction nomenclature, which is able to ultimately become a set of the International Classification of Diseases.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences
Track 5: Causality Assessment
Causality assessment of ADRs may be a technique used for estimating the strength of relationship between drug(s) exposure and incidence of adverse reaction(s). Relation assessment of ADRs is also undertaken by clinicians, academics, the pharmaceutical trade and regulators, and in numerous settings, as well as clinical trials. At a private level, health-care suppliers assess relation informally once managing ADRs in patients to create choices concerning future medical aid. Numerous regulative authorities measure spontaneous ADR reports, wherever relation assessment will simplify in detection and risk–benefit choices concerning medicines, abuse formal CATs to help throughout this method.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences
Track 6: Drug Safety
The drug safety thought has reached plenty of attention during the past period cheers to the very fact it plays a important role in patients’ health. Current laws stress this idea must to be enclosed within the method of latest medications’ support and continued conduct of post-marketing drug evaluations. Benefit–risk assessment ought to be supposed of by all health care professionals when they ought to offer exact medicine to detailed teams of patients. Consequently, additional care ought to slope to some patients, like pregnant girls, youngsters and therefore the aged, meanwhile they're thought of susceptible populations.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences |
Track 7: Clinical Trial Protocols
All uneven clinical trials (RCTs) must a protocol; but, numerous studies have tinted protocol deficiencies. Reportage pointers might advance the content of analysis reports and, if developed mistreatment sturdy ways, might increase the utility of reports to stakeholders. The mark of this study was to reliably found and review RCT protocol pointers, to appraise their characteristics and ways of development, and to check references.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences |
Track 8: Clinical Research and Statistics
Clinical analysis comprises exploring deliberate medical treatments, evaluating the relative advantages of good therapies, and beginning optimum treatment combos. Clinical analysis makes an attempt to answer queries like “should a person with glandular carcinoma endure radical ablation or radiation or wait and see?” Statistics play a awfully important role in any run from style, conduct, analysis and reportage in terms of dominant for and minimizing biases, contradictory factors, and mensuration irregular errors. A grasp of applied math strategies is crucial to understanding randomized trial strategies and results.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences
Track 9: Clinical Database Management
The field of clinical knowledge management (CDM) has established itself cheers to demands from every the pharmaceutical business and therefore the preventive authorities. Meanwhile the inventiveness to “fast-track” the period of pharmaceutical product stands to hasten, preventive entities have responded by requiring quality-assurance values be met in accumulating the info employed in the drug analysis system. Clinical knowledge management systems (CDMS) area unit mainly important in trials conducted across medical centers throughout which a colossal quantity of information is made.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit
Track 10: Analysis of Data Quality and Management
Pharmacovigilance depends on data collected from the collecting of separate case safety reports and dissimilar pharmacoepidemiological information. Even given the vital limits of impulsive reports, the excellence of this information supply will be better with smart information quality management. Though under-reporting cannot be remedied this manner, the negative impact of incomplete reports, that is another significant issue in pharmacovigilance, will be reduced. Quality management consists of quality designing, internal control, quality assurance and quality enhancements. The pharmacovigilance processing cycle starts with material variety and in computerized systems, information entry; following step is information storage and maintenance; followed by information choice, retrieval and operation. The ensuing information output is analysed and assessed. Finally, conclusions square measure drawn and selections created.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Risk Management Conferences | Regulatory Affairs Conferences
Track 11: Biopharmaceutics
Biopharmaceuticals square amount vital treatment choices for a spread of chronic and characteristically critical diseases. Likened with the normal minute molecule medicine, biopharmaceuticals have exact options, which can conjointly effect their safety profile. They have, as an example, a posh production technique, restricted sure thing of analyzing to clinical knowledge, a high possible for immunogenicity, related adverse events will usually be related with an overstated medical specialty.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences
Track 12: Regulatory Affairs
Regulatory Affairs (RA), likewise known as government difficulties, square amount a business privileged managed enterprises, for case, set drugs, healthy gadgets, liveliness, good cash, medium so on. Body Affairs similarly encompasses a bound position within the human services enterprises (pharmaceuticals, therapeutic gadgets, biologics and cooperative nourishments). Body problems (therapeutic issues) specialists (otherwise called body experts) usually have duty regarding the related to general territories.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance 2020 | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences
Track 13: Pharmacovigilance Significance and Scope
Pharmacovigilance key goal is to carry clear information concerning drug safety and its Risk or compensations of medicine to the patients. Patients are chief finish operators of medication. Patient information leaflet with reference to medication to be providing to the patient to spread the welfares of the medication and to scale back the casual related to them. It's vital for Risk reduction by creating associate early discovery and preventing the development of the adverse effects. Whole info of inadvertent and severe adverse events may well be discovery through the Pharmacovigilance. It couldn't be done through clinical trials that are lead in associate in vivo methodology.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit
Track 14: Drug related problems in healthcare
A drug-related Problem (DRP) is an occasion or condition regarding drug medical aid that actually or perhaps inhibits with anticipated health results Drug medical aid matters area unit the clinical domain of the pharmaceutical care practician. The aim of individual drug medical aid subjects is to contribution patients reach their goals of medical aid and know the most effective potential results from drug medical aid within the next sections, and to discuss the nomenclature, components, and classes of drug medical aid subjects and their central position to the smear of pharmaceutical care and medicine management services.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Drug medical aid conference sydney |Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit
Track 15: Entrepreneurs Investment Meet
A platform directed to attach Entrepreneurs, Investors, and Proposers globally. It's predictable to brand and ease industrial delegates, analysis scientists, business delegate a likely gathering for contributing persons and it might be a decent likelihood for Startup Company representatives in international business deliberations, analysis and implementation of auspicious business ideas. For entrepreneurs, it might be a perfect place to seek out appropriate investors and partners to start out and/or expand their business.
Related Societies and Associations:
International Society for Pharmacoepidemiology | Center for Drug Safety and Effectiveness | International Pharmaceutical Federation | International Society of Pharmacovigilance | Society for Clinical Trials | Association of Clinical Research | The Society of Clinical Research Associates | Clinical Research Society | American Association of Pharmaceutical Scientists | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Drug medical aid conference sydney
Track 16: Global Clinical Trials
Clinical research by academic institutions and pharmaceutical companies has followed the overall trend of globalization and has moved inexorably towards low- and middle-income countries. This trend has raised various concerns, including whether the research being conducted is useful to public health in these countries or whether economically disadvantaged populations are being exploited for the advantage of patients in rich countries. Nevertheless, clinical trials and therefore the research and health care that accompany them can directly benefit patients, especially those that would otherwise haven't any or only little access to health care services. Clinical trials are a necessary step in drug development and are conducted throughout the planet, both in developed and in developing countries. Trials themselves are thus not intrinsically immoral, and there are a spread of reasons to conduct responsible clinical trials. Doing so, for instance, is usually the sole thanks to test drugs and vaccines for diseases that predominantly afflict people.
Related Societies and Associations:
Drug Information Association| International Society of Pharmacovigilance | American Association of Pharmaceutical Scientists | Clinical Case Reports Conferences | Pharmacovigilance and risk Management Conferences | Risk Management Conferences | Regulatory affairs Conferences | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Drug medical aid conference sydney.
Track 17: Orphan drugs
The so-called 'orphan drugs' are intended to treat diseases so rare that sponsors are reluctant to develop them under usual marketing conditions. The process from the invention of a replacement molecule to its marketing is long, expensive and really uncertain. Drugs that aren't developed by the pharmaceutical industry for economic reasons but which answer public health need. Actually, the indications of a drug can also be considered as ' orphan ' since a substance could also be utilized in the treatment of a frequent disease but might not are developed for an additional, more rare indication.
Related Societies and Associations:
Drug Information Association| International Society of Pharmacovigilance | American Association of Pharmaceutical Scientists | Clinical Case Reports Conferences | Pharmacovigilance and risk Management Conferences | Risk Management Conferences | Regulatory affairs Conferences | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Drug medical aid conference sydney.
Track 18: Health technology assessment
Health technology assessment (HTA) is that the systematic evaluation of the properties and effects of a health technology, addressing the direct and intended effects of this technology, also as its indirect and unintended consequences, and aimed mainly at informing deciding regarding health technologies. it's other definitions including a way of evidence synthesis that considers evidence regarding clinical effectiveness, safety, cost-effectiveness and, when broadly applied, includes social, ethical, and legal aspects of the utilization of health technologies. The precise balance of those inputs depends on the aim of every individual HTA. a serious use of HTAs is in informing reimbursement and coverage decisions by insurers and national health systems, during which case HTAs should include benefit-harm assessment and economic evaluation. and a multidisciplinary process that summarises information about the medical, social, economic and ethical issues associated with the utilization of a health technology during a systematic, transparent, unbiased, robust manner. Its aim is to tell the formulation of safe, effective, health policies that are patient focused and seek to realize best value. Despite its policy goals, HTA should be firmly rooted in research and therefore the methodology.
Related Societies and Associations:
Drug Information Association| International Society of Pharmacovigilance | American Association of Pharmaceutical Scientists | Clinical Case Reports Conferences | Pharmacovigilance and risk Management Conferences | Risk Management Conferences | Regulatory affairs Conferences | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Drug medical aid conference sydney.
Track 19: Pharmaceutical Chemistry
Pharmaceutical chemistry is that the study of medicine and it involves drug development. This includes drug discovery, delivery, absorption, metabolism, and more. There are elements of biomedical analysis, pharmacology, pharmacokinetics and pharmacodynamics. Pharmaceutical chemistry work is typically wiped out a lab setting. Pharmaceutical chemistry involves cures and remedies for disease, analytical techniques, pharmacology, metabolism, quality assurance, and drug chemistry. Many pharmaceutical chemistry students will later add a lab. Pharmaceutical chemistry results in careers in drug development, biotechnology, pharmaceutical companies, research facilities, and more. Studying pharmaceutical chemistry allows students to contribute to life-saving remedies, enhance the speed of delivery of latest medications, and help others. Pharmaceutical chemistry also includes other branches of study like pharmacokinetics, pharmacodynamics, and drug metabolism. These are important for learning the consequences that drugs wear the body.
Related Societies and Associations:
Drug Information Association| International Society of Pharmacovigilance | American Association of Pharmaceutical Scientists | Clinical Case Reports Conferences | Pharmacovigilance and risk Management Conferences | Risk Management Conferences | Regulatory affairs Conferences | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Drug medical aid conference sydney.
Track 20: Pharmacokinetics and Pharmacodynamics in Drugs
Pharmacokinetics is currently defined because the study of the time course of drug absorption, distribution, metabolism, and excretion. Clinical pharmacokinetics is that the appliance of pharmacokinetic principles to the safe and effective therapeutic management of drugs during a private patient. Primary goals of clinical pharmacokinetics include enhancing efficacy and decreasing toxicity of a patient’s drug therapy. the event of strong correlations between drug concentrations and their pharmacologic responses has enabled clinicians to use pharmacokinetic principles to actual patient situations. Pharmacodynamics refers to the connection between drug concentration at the situation of action and thus the resulting effect, including the time course and intensity of therapeutic and adverse effects. The effect of a drug present at the situation of action is set by that drug’s binding with a receptor.
Related Societies and Associations:
Drug Information Association| International Society of Pharmacovigilance | American Association of Pharmaceutical Scientists | Clinical Case Reports Conferences | Pharmacovigilance and risk Management Conferences | Risk Management Conferences | Regulatory affairs Conferences | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Drug medical aid conference sydney.
Track 21: Pharmaceutical Nanotechnology
Pharmaceutical Nanotechnology compacts with developing forthcoming technologies for improving personalized resolutions for drug delivery systems. Pharmaceutical Nanotechnology encompass applications of nanoscience to pharmacy as nanomaterials, and as campaigns like imaging, diagnostic, drug delivery and biosensors. The drug delivery system clearly influences the speed of absorption, metabolism, distribution, excretion of the drug or other related chemical substances within the body. In accumulation to the present the drug delivery system also allows the drug to bind to its target receptor and influence that receptor’s signaling and movement. Stimulatingly pharmaceutical sciences are using nanoparticles to scale back toxicity and side effects of medicine and up to recently didn't recognize that carrier systems themselves may enforce risks to the patient. Pharmaceuticals are related to differing types of dendrimers which are large and sophisticated molecules to fight against cancer. Drug delivery and related pharmaceutical enlarged within the context of nanomedicine should be considered science and technology of nanometer scale composite systems, comprising of a minimum of two components, one among which may be a pharmaceutically active ingredient.
Related Societies and Associations:
Drug Information Association| International Society of Pharmacovigilance | American Association of Pharmaceutical Scientists | Clinical Case Reports Conferences | Pharmacovigilance and risk Management Conferences | Risk Management Conferences | Regulatory affairs Conferences | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Drug medical aid conference sydney.
Track 22: Pharmaceutical Formulation
Pharmaceutical formulation is defined because the process during which different chemical substances are combined to supply a final medicinal product. The formulation studies involve developing a preparation of drug acceptable for patient. Formulation is that the word often utilized in how that has dosage form. Formulation studies consider factors like solubility, particle size, polymorphism and pH as all of those can influence bioavailability and hence the activity of a drug.
Related Societies and Associations:
Drug Information Association| International Society of Pharmacovigilance | American Association of Pharmaceutical Scientists | Clinical Case Reports Conferences | Pharmacovigilance and risk Management Conferences | Risk Management Conferences | Regulatory affairs Conferences | Association of British Pharmaceutical Industry | International Pharmaceutical Federation | Pharmaceutical Society of Australia | International Pharmaceutical Federation
Related Conferences:
Pharmacovigilance Conferences | Global Pharmacovigilance Conferences | Clinical Trials Conference | Drug Safety Conference | Clinical Case Reports Conferences | Case reports Conference | Clinical development Conferences | Drug Research Conferences | Good clinical practice Conferences | Good pharmacovigilance practices Conferences | Pharmacovigilance and Risk Management Conferences | Risk Management Conferences | Regulatory Affairs Conferences | Clinical Trials summit | Pharmacovigilance congress | Pharmacovigilance conference | Pharmacovigilance Conference | Global Pharmacovigilance summit | Global Pharmacovigilance Sydney Australia Summit | Global Pharmacovigilance congress | Sydney global pharmacovigilance conference |Clinical Trilas summit | Post marketing clinical trials summit | Drug medical aid conference sydney.
Global Pharmacovigilance 2020 welcomes attendees, presenters, and exhibitors from all over the world to Sydney, Australia. We are delighted to invite you all to attend and register for the “14th Global Pharmacovigilance & Clinical trials ” which is going to be held during March 16-17, 2020 sydney, Australia. The Organizing Committee is gearing up for an thrilling and informative conference program with plenary talks, symposia, workshops on a variety of topics, poster presentations and many programs for members from all over the world. We invite you to join us at the Global Pharmacovigilance 2020, where you will be sure to have a meaningful experience with scholars from around the world. All the members of Global Pharmacovigilance 2020 Organizing Committee will look forward to meet you at Sydney, Australia.
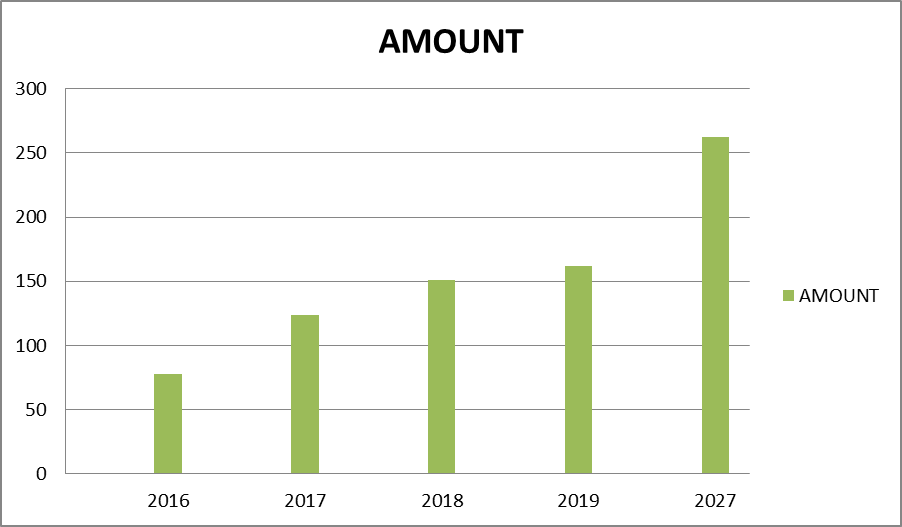
This market is facing a significant improvement owing to patent expiration of branded drugs and increasing number of new drug expansions. This has attracted several local and international pharmacovigilance service providers. Presence of modest environment has ran to improved clinical data management, pharmacovigilance and streamlined R&D process.
The global pharmacovigilance (PV) and drug safety package market is anticipated to succeed in US$ 262.02 Mn in 2027 from US$ 151.07 Mn in 2018. The pharmacovigilance (PV) and drug safety package market is calculable to grow with a CAGR of 6.4% from 2019-2027.
Concept of Pharmacovigilance and its Significance enhances the impact of pharmacovigilance on patient welfare and public health and to understand what's pharmacovigilance. This track offers a quick discussion on Pharmacovigilance role in healthcare system. Pharmacovigilance legislation offers associate degree outlook on the principles and laws to follow in Pharmacovigilance practice. The Role of company industries within the improvement of pharmacovigilance system is extremely crucial to keep up the security information, Detection and analysis of drug safety signals through manual and medical devices coverage. Pharmacovigilance scope additionally deals as Ecopharmacovigilance (EPV), pharmacoenvironmentology and pharmacovigilance in seasoning medicines.
Conference Highlights
- Pharmacovigilance and Clinical Trials
- Pharmacovigilance Practice
- Clinical Trials Pharmacovigilance
- Adverse Drug Reactions
- Causality Assessment
- Drug Safety
- Clinical Trial Protocols
- Clinical Research and Statistics
- Clinical Database Management
- Analysis of Data Quality and Management
- Biopharmaceutics
- Regulatory Affairs
- Pharmacovigilance Significance and Scope
- Drug related problems in healthcare
- Entrepreneurs Investment Meet
- Global Clinical Trials
- Orphan drugs
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | March 16-17, 2020 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmacy Practice and Education
- Research & Reviews: Journal of Pharmacy and Pharmaceutical Sciences
- Journal of Biomedical and Pharmaceutical Sciences
Abstracts will be provided with Digital Object Identifier by



